IMAGEPRO™ PANEL SPEAKS ON CONTRAST AGENTS
Share this article:
There are many legal and policy issues raised by the FDA notice on regulating some imaging agents that meet both the device and drug definition as devices.
The potential impacts are not only to the companies which are market lawful with FDA approved imaging drugs - including contrast media and radiopharmaceuticals - but to healthcare providers, pharmacies, supply chain managers, distributors, and patients. The MarkeTech Group recently surveyed our imagePRO™ panel to gauge their opinions on the recent FDA notice.
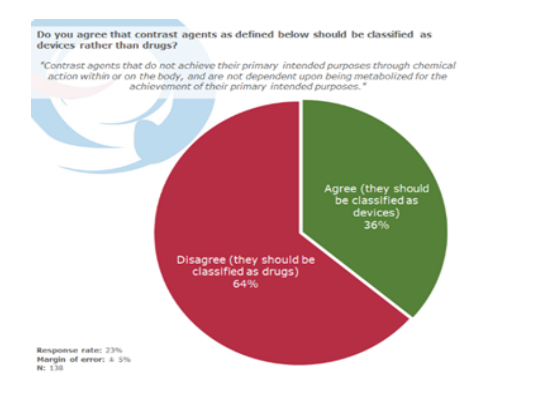
Most respondents disagree that some contrast agents should be classified as devices rather than drugs
When asked whether they agree or disagree that contrast agents as defined below should be classified as devices rather than drugs, 64% indicated that they disagree with the classification definition and pointed out that they should be classified as drugs. While, only 36% agreed with the new classification definition.
Contrast agents that do not achieve their primary intended purposes through chemical action within or on the body, and are not dependent upon being metabolized for the achievement of their primary intended purposes.
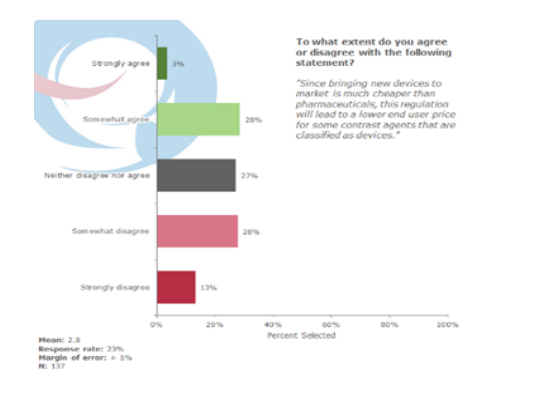
No strong expectation that the end user prices for some contrast agents will reduce
The cost of bringing new devices to market is much cheaper than bringing pharmaceuticals. When asked, 41% of the respondents strongly disagreed or somewhat disagreed that this regulation will lead to a lower end user price for some contrast agents that are classified as devices, while 27% were undecided. Only 31% strongly agreed or somewhat agreed that the end user price for some contrast agents will decrease with the new regulation.
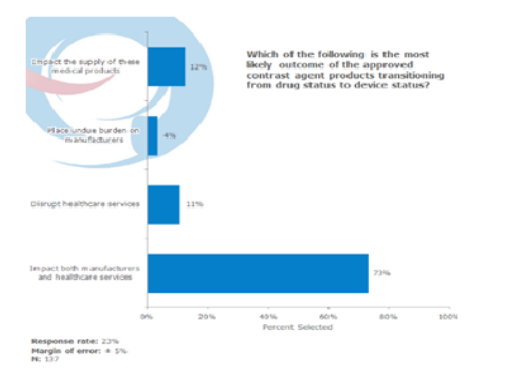
Both manufacturers and healthcare services will likely to be impacted
When we asked respondents about the most likely outcome of the approved contrast agent products transitioning from drug status to device status, 73% stated that this regulation will impact both the manufacturers and the healthcare services. 12% pointed that it will impact the supply of these medical product while another 11% indicated that the outcomes will disrupt the healthcare services. Only 4% thought that there will be undue burden on the manufacturers with FDA’s new regulation.